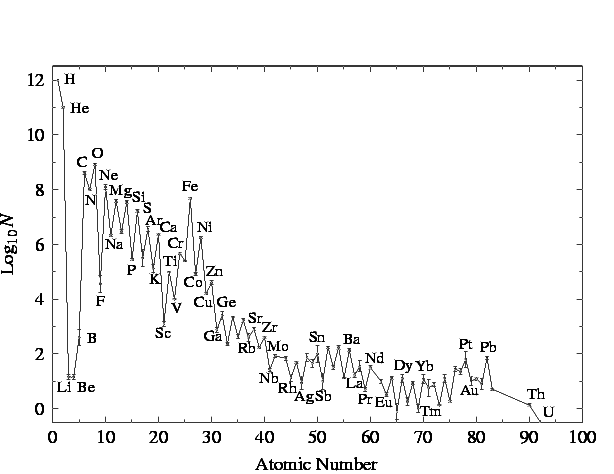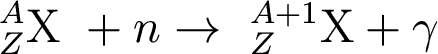
But waitaminnit! Nickle has a higher atomic mass than Iron, and we said that building of heavy elements in stars stopped at Iron. Where'd that nickle come from??
Remember one problem with high mass nucleosynthesis -- the Coulomb barrier. But adding a neutron to a nucleus doesn't have the problems of the Coulomb barrier -- why not?
So neutron capture can take place even at low temperatures, given that we have free neutrons running around. What happens then?
We make an isotope of element X:
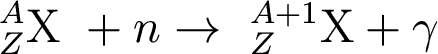
This isotope may or may not be stable. If it is, it's happy. If it isn't, it undergoes beta decay:
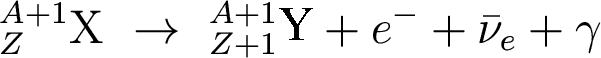
And we have a new element Y with Z+1 protons!
How long does beta decay take to occur? It depends on the isotope -- some happen fast (seconds), some happen slow (days).
If beta decay takes a long time, you can actually add a neutron to an unstable isotope before it decays, making yet a different isotope.
We define two types of neutron addition processes:
Slow (s-)process: neutrons are added slowly, after isotopes have had time to beta decayWhere would the r-process occur?
Rapid (r-)process: neutrons are added fast, one after the other, until beta decay finally occurs
In this way the heavy elements ("past the iron peak") are formed: