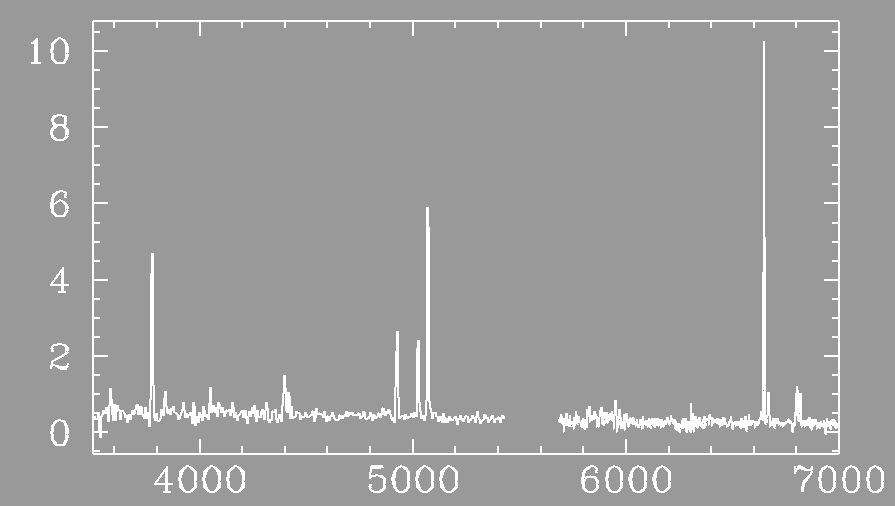
Example: A star-forming gas cloud in a distant galaxy (courtesy Stacy McGaugh)
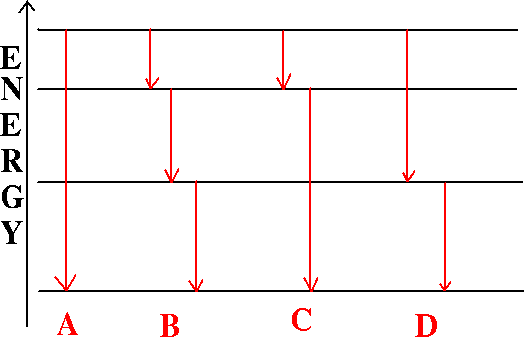
1. A hot dense gas or solid object produces a continuous spectrum with no dark spectral lines.
It radiates as a black body of temperature T, according to the Planck function.2. A hot diffuse gas produces bright emission lines.
Emission lines occur when electrons move from a higher orbit to a lower orbit in the atoms, emitting a photon of exactly the right wavelength that corresponds to the energy difference in the orbits.3. A cool diffuse gas in front of a continuous source produces dark lines in the continuous spectrum.Example: A star-forming gas cloud in a distant galaxy (courtesy Stacy McGaugh)

Light of a wavelength that corresponds to energy differences in atomic orbits will be absorbed, boosting the electrons to a higher orbit.
Things which affect spectral lines:
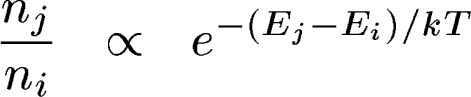
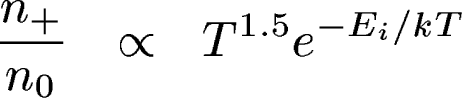
Example: Balmer absorption lines
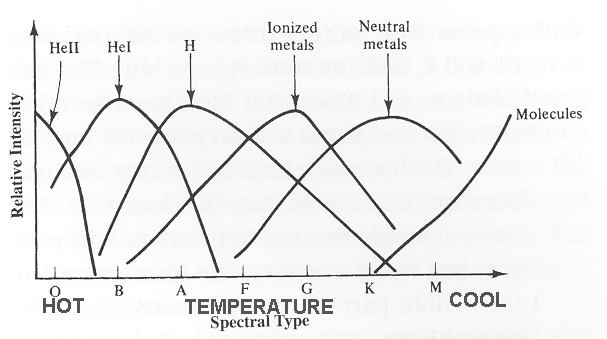
| Similar things
happen for all atoms and molecules, depending on their ionization and
excitation energies. So we see different lines in stars of different temperatures, even if they have the same chemical composition. |
 |